Even as the economy continues to boom, medical device manufacturers face unending challenges from supply chain issues, rising materials and energy costs, and workforce constraints. Those forces are mostly out of your control. But internally, where you do have control, there are ways to increase your operational and market competitiveness. For manufacturers in this industry, medical device inventory management is both a critical strength and a great place to begin looking for opportunity.
Inventory is an accepted cost of doing business for manufacturers and is often the largest cost category. Since medical devices are typically high-value products, any efficiencies or cost reductions can quickly contribute to the bottom line. But it’s not as simple as cutting back on the number of products on warehouse shelves. Medical device inventory management must consider strict industry regulations, detailed track and trace requirements, and auditability down to the part and component level. Furthermore, those medical device inventory management requirements extend beyond your walls and warehouses to include raw material and suppliers as well as pinpoint tracking of products at contract manufacturers, at distributors, in sales reps’ trunks, and at medical facility storage locations.
The Rising Costs of Medical Device Inventories
Cost is the crux of medical device inventory management, especially for a market that is expected to reach $800 billion by the end of the current decade. Optimizing to minimize excess inventory without risking stockouts is the balancing act. Simply cutting inventory isn’t a viable approach. To win, says KPMG, medical device manufacturers must shift from a focus on pure costs to optimizing around “smart value.” Otherwise, your medical device inventories are working capital that’s not working.
Medical device inventory management must also consider the intersection of advancing technologies, increasing costs, slower supply chains, and industry regulations. Studies suggest that medical device manufacturers have 150 days of inventory in the field, on average. Some have up to 400 days’ worth of inventory on hand. As your days on hand increase, so do the risks that your stores of medical devices may become obsolete as technology advances adding another component of complexity for suppliers and storage facilities scattered around your customers’ and supply chain.
Connecting your planning more tightly with your customers’ demand plans will result in better customer service and faster order fulfillment. But you need real-time tools to make faster decisions based on accurate, timely data. That’s difficult if you’re relying on spreadsheets and email to track and manage inventories. Those manual processes are inefficient, slow, invite errors, and risk obsolete products sitting on customers’ shelves.
How to Modernize Your Medical Device Inventory Process
Modernizing your medical device inventory management processes is a proven first step towards both cutting costs and increasing effectiveness. Digital transformation brings automation to speed processes, improve visibility, and increase accuracy. That then leads to better optimizations that reduce overstocks, highlight expired products, better utilize warehouse space, and give sales reps real-time information on inventories, lead times, and more.
Connecting modern medical device inventory management to the rest of your business can also help improve workforce scheduling, inform engineering on product usage, and streamline the entire quote-to-cash process. Deeper tracking down to a product’s lot and serial number enable full track and trace as products move from production through inventory and customer receipt. Audit trails can then integrate with quality control to satisfy regulatory compliance requirements. And, if a recall occurs, you can quickly report where products are located and which customers may be affected.
Learn the 5 benefits of ERP automation for medical device manufacturers.
For finance and accounting teams, more accurate inventory management also enables usage of standard cost, actual cost, or FIFO costing, and helps your company manage costs at any level. That inventory information then empowers workers to make proper material choices to reduce waste caused by obsolescence, increase inventory turns, and drive higher profitability.
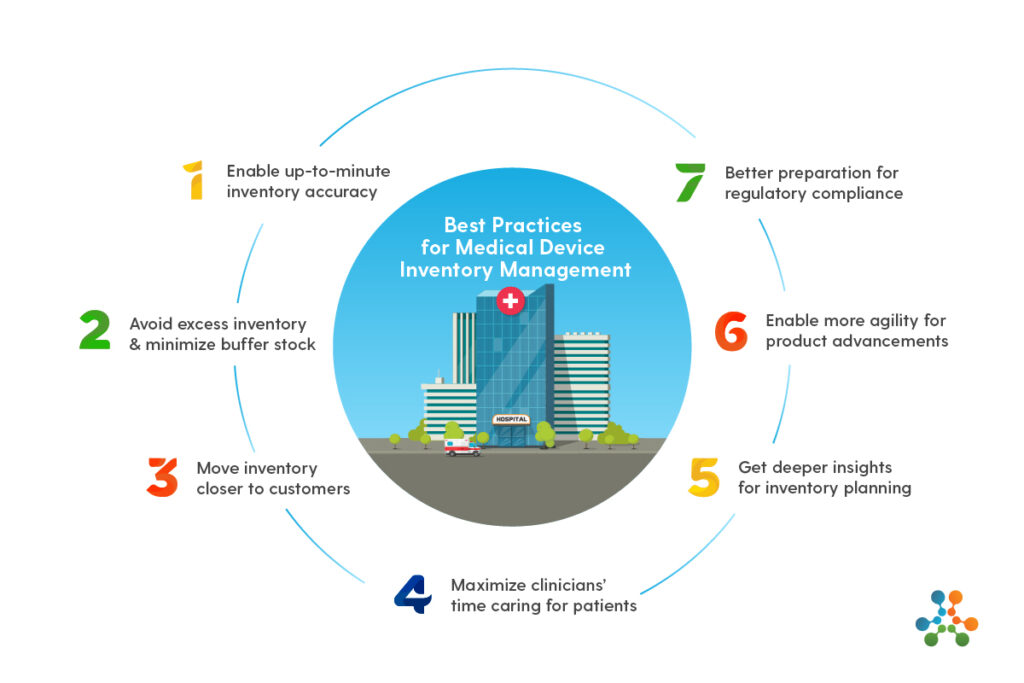
7 Best Practices for Medical Device Inventory Management
So now you know the benefits of modernizing your medical device inventory management. What’s next? Here are 7 critical best practices you can deploy to improve your capabilities.
1. Enable up-to-the-minute inventory accuracy
As products make their way from production to customers, raw materials, component, and finished goods inventories constantly change. But what is where? And what’s going to be there tomorrow? Gaining real-time access to inventory movements across your business increases efficiencies and gives you complete visibility into all item, kit, and stock volumes across all locations. That helps you better manage your medical device inventories and adapt to any changes in the market or your business.
2. Avoid excess inventory and minimize “buffer stock”
Tighter control over medical device inventories helps you create more accurate and optimal production plans and schedules. You only want to produce what’s expected otherwise any extra locks up cash that could have been invested elsewhere. Better inventory management enables you to produce exactly what’s needed instead of overproducing. As forecast and inventory data become more accurate, safety stock levels can be reduced.
3. Move inventory closer to customers
Good medical device inventory management includes optimizing inventory by location. Modern systems are flexible in that they allow you to track inventory across different types of locations. If you have available warehouses, distributors, or space on-site at customer facilities, you can stage products closer to demand to reduce order lead times, increase customer satisfaction, and accelerate invoicing and cash flow.
4. Maximize clinicians’ time caring for patients
Putting undue inventory burden on customers’ workforces pulls them away from their true duties. Today’s medical device customers are hyper focused on reducing costs while increasing the quality of patient care. That translates to efficiency. Inaccurate medical device inventory management creates inefficiencies and avoidable costs for health care providers. That could quickly become a customer satisfaction issue and drive customers to competitors that have better inventory management practices.
5. Get deeper insights for inventory planning
More visibility is always a good thing, especially for expensive medical device inventories. Usage trends and data gives planners more confidence when making decisions on inventory purchases. Visibility highlights where stock is too high or too low so teams can quickly act. But that data–when it’s a single source of truth for your entire company–can also be used upstream to help sales, logistics, engineering, procurement, and other teams better understand usage, seasonality, customer preferences, and more. As inventory information becomes more accurate and trusted across the organization, customer service levels will increase as order fill rates and lead times are reduced.
6. Enable more agility for medical device product advancements
Technology is always advancing. Medical device inventory management must take that into account so currently shipping products can be sold first to make room for new and updated products. Accurate information on product updates, reused components, component revision effectivity, and more gives inventory managers the insight to make smart changes when new and updated parts or products enter the pipeline.
7. Be better prepared to accommodate regulatory compliance
Regulations require medical device manufacturers to maintain detailed quality and product information throughout the supply chain. Real-time data management is the most effective way to bring speed and accuracy to your regulatory compliance processes. Manual, paper-based methods simply can’t provide the same levels of accuracy for product histories, full traceability, audit trails, and quality control.